Researcher Develops Completely Explosion-Proof Lithium Metal Battery With 2X Power Of Lithium Ion
It seems that we're constantly hearing about promising new battery technologies and eventually one of them will stick. Mike Zimmerman, a professor at Tufts University and founder of Ionic Materials, hopes that his remarkably resilient ionic battery technology will be the one that does. At a glance, his ionic battery technology appears to a legitimate shot at finally pushing the category forward in a significant way.
The reason scientists and researchers pay so much attention to battery design is because today's lithium-ion units have several downsides. As we saw recently with Samsung's Galaxy Note 7 recall, they can overheat and catch fire. Even when they work correctly, lithium-ion batteries degrade over a relatively short time as they go through recharge cycles, and they don't last all that long to begin with.
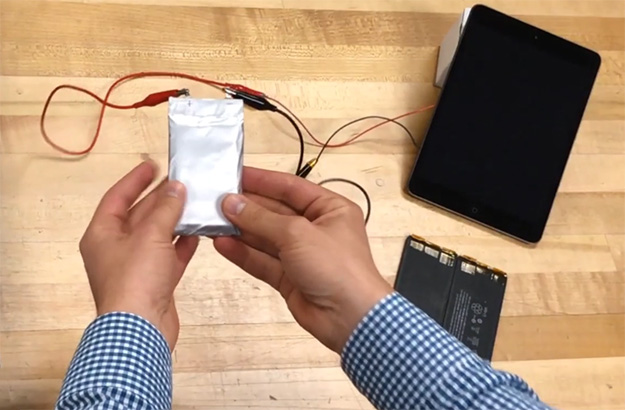
Mike Zimmerman's Ionic battery doesn't overheat or catch fire like lithium-ion batteries can.
If you were to take apart a lithium-ion battery, you'd find a positive electrode called the anode and a negatively charged electrode called the cathode. There's a thin sheet with microscopic pores called a separator that sits between the anode and cathode. Everything else is filled up with liquid, or electrolyte.
Charging the battery causes positively charged ions to flow through the liquid from the negative side to the positive side. As you use the battery, the ions flow in the opposite direction. It's a serviceable solution, but the electrolyte is extremely flammable and sensitive to being poked—they can explode when pierced. This is called thermal runaway, which is what the Galaxy Note 7's batteries experienced.
Zimmerman's ionic battery trades the flammable liquid for a piece of plastic film to serve as the electrolyte. It isn't prone to overheating and catching fire. You can even take a lighter to it and it still won't catch fire. The same goes for piercing it, cutting it, or otherwise destroying the battery in some other physical manner.
This is something Zimmerman has demonstrated by connecting an LED panel to his ionic battery. Even as he bends and cuts the battery, the LEDs remain lit. He's also done this while powering an iPad with his battery, as shown above.
That's not the only benefit. Unlike lithium-ion batteries, Zimmerman's ionic batteries use actual lithium-metal, which can store twice as much power. Lithium-ion batteries don't contain lithium-metal because they're even more prone to overheating and exploding than lithium-ion, but that risk is removed by Zimmerman swapping out the liquid electrolyte for a solid.
Will this be the next big thing in batteries? We don't know, though Zimmerman says that electronic companies have paid him a visit at his Ionic Materials facility in Woburn, MA. Zimmerman admits there is still a lot of reliability testing to be done and it will be difficult to scale his operations, but he's confident it can and will be accomplished.
The reason scientists and researchers pay so much attention to battery design is because today's lithium-ion units have several downsides. As we saw recently with Samsung's Galaxy Note 7 recall, they can overheat and catch fire. Even when they work correctly, lithium-ion batteries degrade over a relatively short time as they go through recharge cycles, and they don't last all that long to begin with.

Mike Zimmerman's Ionic battery doesn't overheat or catch fire like lithium-ion batteries can.
If you were to take apart a lithium-ion battery, you'd find a positive electrode called the anode and a negatively charged electrode called the cathode. There's a thin sheet with microscopic pores called a separator that sits between the anode and cathode. Everything else is filled up with liquid, or electrolyte.
Charging the battery causes positively charged ions to flow through the liquid from the negative side to the positive side. As you use the battery, the ions flow in the opposite direction. It's a serviceable solution, but the electrolyte is extremely flammable and sensitive to being poked—they can explode when pierced. This is called thermal runaway, which is what the Galaxy Note 7's batteries experienced.
Zimmerman's ionic battery trades the flammable liquid for a piece of plastic film to serve as the electrolyte. It isn't prone to overheating and catching fire. You can even take a lighter to it and it still won't catch fire. The same goes for piercing it, cutting it, or otherwise destroying the battery in some other physical manner.
This is something Zimmerman has demonstrated by connecting an LED panel to his ionic battery. Even as he bends and cuts the battery, the LEDs remain lit. He's also done this while powering an iPad with his battery, as shown above.
That's not the only benefit. Unlike lithium-ion batteries, Zimmerman's ionic batteries use actual lithium-metal, which can store twice as much power. Lithium-ion batteries don't contain lithium-metal because they're even more prone to overheating and exploding than lithium-ion, but that risk is removed by Zimmerman swapping out the liquid electrolyte for a solid.
Will this be the next big thing in batteries? We don't know, though Zimmerman says that electronic companies have paid him a visit at his Ionic Materials facility in Woburn, MA. Zimmerman admits there is still a lot of reliability testing to be done and it will be difficult to scale his operations, but he's confident it can and will be accomplished.

