Forget The Energizer Bunny, Your Next Battery Could Be Made Of Crab Shells And Squid

Scientists have found a surprising source for a sustainable battery electrolyte material – crab shells. This naturally abundant and renewable source, commonly discarded, can yield chitin, which is simple to turn into the chitosan electrolyte material. As our headline suggests, crab shells aren’t the only natural source for Chitin, other crustacean shells contain it (e.g. lobster, crayfish, shrimp) as well as squid and some fungi.
Batteries are increasingly important in the energy supply chain. Many renewable and sustainable sources provide abundant electricity, but their peak production timing can’t be controlled. For example, we can’t make the sun shine or wind blow harder. This makes renewables harder to integrate into the energy grid compared to fossil fuel or nuclear energy sources, which can easily adjust power output to coincide with demand patterns. Batteries are thus going to play an important buffering role in our future, unless some other renewable / sustainable energy source emerges which isn’t reliant on times, tides or winds etc.
In a paper dubbed ‘A sustainable chitosan-zinc electrolyte for high-rate zinc-metal batteries’, lead author Liangbing Hu, the Director of the University of Maryland's Center for Materials Innovation, talked about his team’s tests with crab shell sourced electrolyte materials and the possible implications of this discovery.
“Vast quantities of batteries are being produced and consumed, raising the possibility of environmental problems,” noted Hu. He went on to list some of the environmentally undesirable constituents of Li-ion batteries, which once past their service life will take “hundreds or thousands of years to degrade,” and then cease being an environmental hazard. In contrast, the chitosan electrolyte is readily biodegradable. Hu and his team assert that this renewable electrolyte material can be broken down by microbes in approximately five months. Moreover, choosing Zinc as the battery metal component means that this relatively benign and naturally abundant metal can be recycled after the electrolyte has broken down – far preferable to lead or lithium.
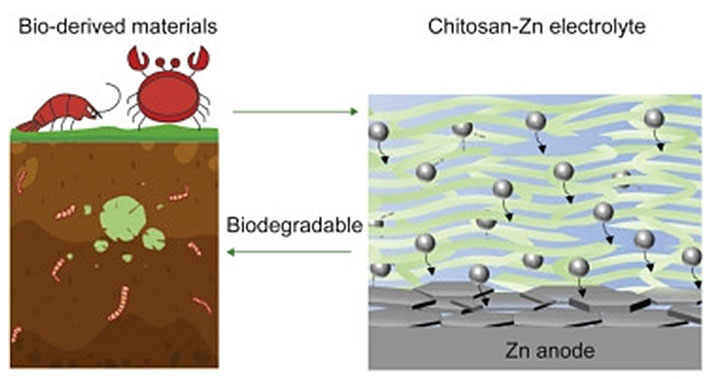
Hu shared some promising performance figures for the zinc and chitosan battery. The chemistry promises a long-lasting rechargeable system, with little degradation over its life. After 1,000 battery cycles, users can still expect a zinc and chitosan battery to be 99.7% efficient, claimed the scientist.
With the above success behind them, Hu and his team intend to continue working on improving other aspects of battery chemistry, and the production of batteries, with an eye on avoiding negative impacts on the environment.

